|
| |
Laboratory Investigation (2004) 84, 1439–1450,
advance online publication, 27 September 2004; doi:10.1038/labinvest.3700177
Diego Cadavid1,2,
Yunhong Bai1,2,
Emir Hodzic3,
Kavitha Narayan1,
Steven W Barthold3
and Andrew R Pachner1
- 1Department of Neurology and Neuroscience,
UMDNJ-New Jersey Medical School, Newark, NJ, USA
- 2Center for the Study of Emerging Pathogens,
UMDNJ-New Jersey Medical School, Newark, NJ, USA
- 3Center for Comparative Medicine, University of
California at Davis, Davis, CA, USA
Correspondence: Dr D Cadavid, MD, Department of Neurology
and Neuroscience, UMDNJ-New Jersey Medicine School, 185 South Orange
Avenue, MSB H506, Newark, NJ 07103, USA. E-mail: Cadavidi@umdnj.edu
Received 9 March 2004; Revised 13 July 2004;
Accepted 16 July 2004; Published online 27 September 2004.
Top of page
Abstract
To investigate cardiac involvement in the non-human
primate (NHP) model of Lyme disease, we inoculated 39 adult Macaca
mulatta with Borrelia burgdorferi sensu stricto strains N40
(BbN40) by needle (N=22, 14 immunocompetent (IC), seven permanently
immunosuppressed (IS), and four transiently immunosuppressed (TISP)) or by
tick-bite (N=4, all TISP) or strain 297 (Bb297) by needle (N=2
IS), or with B. garinii strains Pbi (N=4, 2 TISP and 2 IS),
793 (N=2, TISP) or Pli (N=2, TISP). Five uninfected NHPs
were used as controls. Infection and inflammation was studied in the
hearts and the aorta removed at necropsy 2–32 months after inoculation
by (1) H&E and trichrome-staining; (2) immunohistochemistry and
digital image analysis; (3) Western blot densitometry; and (4) TaqMan RT-PCR.
All NHPs inoculated with BbN40 became infected and showed carditis at
necropsy. The predominant cells were T cells, plasma cells, and
macrophages. There was increased IgG and IgM in the heart independent of
immunosuppression. The B-cell chemokine BLC was significantly increased in
IS-NHPs. There was increased deposition of the complement membrane attack
complex (MAC) in TISP and IS-NHPs. The spirochetal load was very high in
all BbN40-inoculated IS-NHPs but minimal if any in IC or TISP NHPs.
Double-immunostaining revealed that many spirochetes in the heart of
BbN40-IS NHPs had MAC on their membranes. We conclude that carditis in
NHPs infected with B. burgdorferi is frequent and can persist for
years but is mild unless they are immunosupressed.
Keywords:
Borrelia burgdorferi, non-human primates,
heart, digital image analysis, plasma cells, BLC, membrane attack
complex
Lyme borreliosis is a systemic disease caused by infection
with the spirochete Borrelia burgdorferi.1
It is currently the most common arthropod-borne disease in the United
States, where thousands of cases are reported to the Center for Disease
Control every year.2 At least three
genospecies pathogenic to humans have been characterized: B.
burgdorferi sensu stricto, B. afzelii, and B. garinii.
Only B. burgdorferi sensu stricto is endemic in North America,
while all three genospecies are endemic in Europe. The organs most often
affected are the skin, the joints, the heart, and the central and
peripheral nervous system.
Cardiac manifestations of B. burgdorferi infection
occur in up to 8% of patients.1, 3
Clinically, Lyme carditis is typically characterized by varying degrees of
intermittent atrioventricular block occurring within weeks of infection
with B. burgdorferi, a median of 21 days from the onset of erythema
migrans. Temporary cardiac pacing may be required in up to one-third of
cases. Myocarditis and/or pericarditis may occur and also mild left
ventricular dysfunction that rarely can be fatal.1
Cardiomyopathy has been associated with B. burgdorferi infection in
Europe but not in the United States.4
Our laboratory has been studying the pathogenesis of Lyme
borreliosis using non-human primates (NHPs) for several years.5,
6, 7, 8, 9,
10 During infection of immunosuppressed NHPs
inoculated with the sensu stricto strain N40 of B. burgdorferi
(BbN40), we found that the heart had the most severe injury of all tissues
examined and one of the highest spirochetal loads.6
The goal of the present study was to characterize the full spectrum of
Lyme carditis in the NHP model using different strains of B.
burgdorferi and degrees of immunosuppression during both short- and
long-term infection and after syringe or tick-inoculation. The results
showed that cardiac inflammation was a consistent finding in all NHPs
inoculated with BbN40 but was mild unless the animals were
immunosuppressed.
Top of page
Materials and methods
Borrelia Strains
The following Borrelia strains were used for these
experiments: B. burgdorferi sensu stricto strains N40 (BbN40) and
297 (Bb297); and B. burgdorferi subspecies garinii strains Pli (BgPli),
793 (Bg793), and Pbi (BgPbi). BbN40 is a North American tick-isolate,11
Bb297 is a CSF isolate from a patient in Connecticut.12
BgPbi and BgPli are CSF isolates from Europe,13
and Bg793 is a tick isolate from Europe.
Animals Inoculation and Necropsy
A total of 39 adult Macaca mulatta were
inoculated intradermally with different strains of B. burgdorferi
sensu stricto or garinii as follows: BbN40 by needle (N=22)
or by tick-bite (N=4); Bb297 by needle (N=2); or BgPbi (N=4),
Bg793 (N=2) or BgPli (N=4) all by needle. The methods for
tick or needle inoculation and for immunosuppression have been reported
before5 as well as results for infection
of all the immunosuppressed animals inoculated with BbN40, Bb297, and
garinii strains. The hearts from five NHPs that were uninfected were
used as negative controls.
Histology
Tissues from all organs were processed for
histology by routine formalin-fixation and embedding in paraffin or
snap-frozen in cryomatrix (Shandon) in isopentane chilled to less than
-140°C in liquid nitrogen. Paraffin sections were cut at 5  m
and cryomatrix sections at 8 m
and cryomatrix sections at 8  m.
Inflammation was assessed by examination of hematoxylin and eosin
(H&E) staining. To compare the severity of inflammation in heart,
H&E-stained sections were graded by a masked examiner for the
severity of inflammation per m.
Inflammation was assessed by examination of hematoxylin and eosin
(H&E) staining. To compare the severity of inflammation in heart,
H&E-stained sections were graded by a masked examiner for the
severity of inflammation per  40 microscopic field as follows: absent inflammation=0; 1–3
foci=1+(minimal); 4–10 foci=2+(mild); 11–20 foci=3+(moderate); and
more than 20 foci: 4+(severe).
40 microscopic field as follows: absent inflammation=0; 1–3
foci=1+(minimal); 4–10 foci=2+(mild); 11–20 foci=3+(moderate); and
more than 20 foci: 4+(severe).
Enzyme-Linked Immunosorbent Assay and Immunoblot
Serum enzyme-linked immunosorbent assay (ELISA)
and Western blots were performed as described.5,
7, 14 The strain used for
preparation of most Western blots was B. burgdorferi sensu stricto
strain CB, an isolate from an erythema migrans lesion from Valhalla, NY.
ELISA and immunoblots were repeated for the garinii-inoculated NHPs
using antigens from B. garinii strains.
Immunohistochemistry and Image Analysis
Immunohistochemistry and digital image analysis
were performed as previously described.6, 7
Antigen retrieval by microwave heating (Dako's target retrival solution,
Code No. S1699) or protease digestion (P-6911, Sigma Protease) was used
with formalin-fixed tissues. Rabbit polyclonal antibody antihuman IgG (Dako's
A0423), IgM (Dako's A0425), C1q (Dako's A0136), and CD3 (T cell marker,
Dako's A0452), mouse monoclonal antibody anti-P63 (plasma cell marker,
Dako's M7077), Ham56 (monocyte/macrophage marker, Dako's M0632), C9
neoepitope (MAC) (Dako's M0777), and goat anti-human BLC/BCA(R&D
AF801) were used as primary antibody. Recombinant human BLC/BCA peptide
(R&D, 801-CX) was used for blocking assays of the anti-BLC antibody
to confirm its specificity. Primary antibody for detection of B.
burgdorferi was hyperimmune serum from a rabbit persistently
infected with B. burgdorferi strain N40.15
For negative controls, duplicate sections on each glass slide were
incubated with affinity-purified nonspecific antibody (Sigma) matched
for concentration, species, and isotype. Spleen or lymph node tissues
were used as positive controls for markers of inflammation.
The intensity and extent of the immunohistochemical
stains were compared by digital image analysis with Image-Pro Plus
software 4.1 (Media Cybernetics). For this, a masked examiner (YB) took
4–6 digital images at  40,
40,  100, or
100, or  200- magnification. The mean (s.d.) sum area (in square microns) and sum
optical density (in arbitrary units) were determined and compared
between groups. The intensity of immunostaining for some markers (CD3,
P63, and C1q) was compared manually by semiquantitation of the intensity
of the staining as absent (0), mild (1), moderate (2), or severe (3) by
a masked examiner.
200- magnification. The mean (s.d.) sum area (in square microns) and sum
optical density (in arbitrary units) were determined and compared
between groups. The intensity of immunostaining for some markers (CD3,
P63, and C1q) was compared manually by semiquantitation of the intensity
of the staining as absent (0), mild (1), moderate (2), or severe (3) by
a masked examiner.
Immunofluorescence Staining
C5b-9 (MAC) and B. burgdorferi double
immunofluorescence staining was performed with fluorescein
isothiocyanate (FITC)-conjugated (Sigma, F0382) anti-rabbit and
tetramethyl rhodamine isothiocyanate (Tritc)-conjugated anti-mouse goat
polyclonal antibody (Sigma, T6528) at 1/250 dilution. Adobe Photoshop
V7.0 software was used to merge single color images.
PCR
Total RNA was extracted with TRIzole reagent (Life
technologies) from 100 mg NHP tissue blocks. The reverse
transcription (RT) was performed in 20- l
reaction volumes. Taqman RT-PCR for the 16S rRNA of Borrelia spp.
was performed as described.5, 7
PCR-ELISA for the OspA or OspB B. burgdorferi genes was performed
as described.6 All assays were run in
triplicate. l
reaction volumes. Taqman RT-PCR for the 16S rRNA of Borrelia spp.
was performed as described.5, 7
PCR-ELISA for the OspA or OspB B. burgdorferi genes was performed
as described.6 All assays were run in
triplicate.
Immuno Dot-Blot
Immuno dot-blot was performed as described.7
Protein concentration was determined in the supernatant by the BCA
protein assay (Pierce). Dot-blots were prepared by spotting 0.02–0.2  g
in duplicate from each protein extract to polyvinylidene difluoride
membranes (Millipore). The primary antibodies were rabbit polyclonal
anti human IgG (Dako) or IgM at a 1:5000 dilution. The secondary
antibody was alkaline-phosphatase-conjugated goat anti-rabbit IgG
(Sigma). After incubation in fluorescence substrate ECF (Amersham's
RPN5785) for 5 min, the membranes were scanned with the Typhoon
8600 (Amershan Pharmacia Biotech Inc.). Results were analyzed by
densitometry using Image-Quant Software and expressed as mean (s.d.). g
in duplicate from each protein extract to polyvinylidene difluoride
membranes (Millipore). The primary antibodies were rabbit polyclonal
anti human IgG (Dako) or IgM at a 1:5000 dilution. The secondary
antibody was alkaline-phosphatase-conjugated goat anti-rabbit IgG
(Sigma). After incubation in fluorescence substrate ECF (Amersham's
RPN5785) for 5 min, the membranes were scanned with the Typhoon
8600 (Amershan Pharmacia Biotech Inc.). Results were analyzed by
densitometry using Image-Quant Software and expressed as mean (s.d.).
Statistical Analysis
For digital image analysis, differences in mean
sum density or area were compared for statistical significance using
nonparametric tests (Mann–Whitney test) with the SPSS software version
10. The P-values lower than 0.05 were considered significant. For
immuno dot-blot, results were compared for significance by Student's t-test.
Top of page
Results
Animal Infections
A total of 39 adult NHPs inoculated with different
strains of Borrelia burgdorferi were used for these studies. The
results of infection of all animals that were immunosuppressed have been
published.5 Table 1
lists the results of infection of all immunocompetent NHPs inoculated
intradermally with B. burgdorferi strain N40. None of the NHPs
developed erythema migrans and no signs of neurological disease were
apparent to care takers. Serial examination of cerebrospinal fluid (CSF)
failed to show any evidence of CSF-leukocytosis. Viable spirochetes were
identified by tissue culture at necropsy only in immunosuppressed
animals.
Antibody Response
ELISA on necropsy sera showed that all
immunocompetent NHPs inoculated with BbN40 developed specific antibody (Table
1). In contrast, only one out of two NHPs inoculated with Bb297 and
four out of eight NHPs inoculated with B. garinii strains had
detectable anti B. burgdorferi antibody by ELISA and at low
titers.5 The immunoblot for all
inoculated NHPs have been reported.5, 16,
17 Table 1 also
summarizes the immunoblot results for the BbN40-inoculated
immunocompetent NHPs: all that were tested had positive Lyme IgG WB at
necropsy and none had positive IgM WB when examined later than 6 months
after inoculation. All WB from garinii-inoculated NHPs were negative
even when tested with homologous sonicates,5
an indication that NHPs are resistant to syringe inoculation of garinii
strains of Lyme disease borrelias.
Inflammation in the Heart
The aorta and the atrium, ventricle and apex of
the hearts from all NHPs were examined for the presence of inflammation
(carditis) by light microscopy of H&E-stained paraffin and frozen
sections (Figure 1a). The results showed
inflammation in at least one cardiac tissue block from all but four of
the 39 inoculated NHPs. No carditis was observed in any of the five
uninfected controls. To compare the frequency and severity of
inflammation, we calculated a mean sum inflammatory score (see Materials
and methods) (Table 2). The highest mean sum scores
were 0.31, 0.30, and 0.28 for the BbN40-inoculated TISP-tick, IS, and
TISP-needle NHPs, respectively. The mean sum inflammatory score was also
increased in the IC-short-term and long-term groups (0.20 and 0.26) and
in the Bg793 garinii group (0.28) (not shown). Trichrome staining of the
ventricles of BbN40-inoculated NHPs showed most had increased collagen
deposition compared with uninfected controls (Figure 1b).
The mean (s.d.) sum density score for collagen per  40 microscopic field was 2638 (2231), 12 327 (11 351), 13 762
(8176), and 21 246 (24 807) for uninfected controls, IS, TISP-tick,
and IC-short term NHPs, respectively (P-value <0.01 for all
groups compared with uninfected controls).
40 microscopic field was 2638 (2231), 12 327 (11 351), 13 762
(8176), and 21 246 (24 807) for uninfected controls, IS, TISP-tick,
and IC-short term NHPs, respectively (P-value <0.01 for all
groups compared with uninfected controls).
 Full
table Full
table
Light microscopic examination indicated that the predominant
inflammatory cells were mononuclear cells, many with morphological features of
plasma cells. To further characterize the inflammatory infiltrate we did
immunostaining for T cells (CD3, Figure 1c), plasma cells
(P63, Figure 1d), and macrophages (Ham56) and compared
them manually (for T cells and plasma cells, Table 3) or
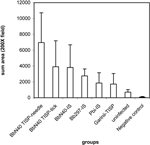
by digital image analysis (for macrophages, Figure 2). The
results showed that there were more T cells and plasma cells in the IS group,
followed by the short-term-IC group. The extent of macrophage infiltration was
higher in short-term-IC, IS, TISP-tick, and TISP-needle NHPs than in
uninfected controls or the long-term-IC group (P-value compared with
uninfected controls was <0.05 for short-term-IC and <0.01 for TISP-needle
and TISP-tick).
Spirochetal Localization and Numbers
To investigate the localization of spirochetes in the
heart, we examined tissue sections immunostained with anti-B. burgdorferi
specific antibody. Spirochetes were found in the aorta and the heart from
BbN40 or Bb297 inoculated IS-NHPs (Figure 1, panels e and
f). Some areas had very large numbers of spirochetes, as many as 5–10 per  400 microscopic field (Figure 1f). Their localization was
predominantly in connective tissue in the aorta and the heart atrium and
ventricle (endocardium, pericardium, and epicardium). In no case, they
appeared to be intracellular in macrophages or cardiac myocytes.
400 microscopic field (Figure 1f). Their localization was
predominantly in connective tissue in the aorta and the heart atrium and
ventricle (endocardium, pericardium, and epicardium). In no case, they
appeared to be intracellular in macrophages or cardiac myocytes.
To investigate the presence of B. burgdorferi in
tissues at necropsy, we used OspA or OspB PCR-ELISA or Borrelia 16S rRNA
TaqMan RT-PCR (Table 4). The results showed that in all
tissues examined from IC or TISP NHPs the signal was either negative or only
weakly positive, with inconsistent results when multiple areas from the same
heart were examined (not shown). In contrast, the TaqMan RT-PCR detected large
numbers of spirochetes in the heart of BbN40-inoculated IS-NHPs (Table
5).
Antibody Deposition
Plasma cells were common in the heart from all
BbN40-inoculated NHPs. Since the production of immunoglobulin is the primary
function of plasma cells, we next looked for the presence of antibody in
hearts from BbN40-inoculated NHPs. Light microscopic examination revealed
extensive deposition of IgG and IgM in the membranes of cardiac myocytes and
blood vessels and in the connective tissue throughout the heart and the aorta
(not shown). Digital image analysis showed significantly increased deposition
of IgG in all BbN40-inoculated NHPs compared with uninfected controls (P<0.001)
(Figure 3a). There were also significant differences in
IgM deposition (Figure 3b). IS and to a lesser extent
TISP-NHPs but not the short-term-IC group had significantly increased IgM
deposition compared with uninfected controls (P<0.01).
To confirm if the hearts from IS-NHPs had higher antibody
deposition than the TISP groups, we did dot-blot densitometry in whole-protein
extracts from ventricles (Table 6). The results confirmed
that there was significantly more IgM in IS than in both TISP NHP groups. It
also revealed higher IgM in the TISP-needle compared with the TISP-tick and
higher IgG in the TISP-needle than in the other two groups.
Complement Deposition
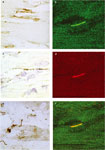
Immunohistochemistry showed deposition of the first
component of the complement cascade (C1q) in the heart from some inoculated
NHPs (Figure 4a). The localization was predominantly
membrane bound, perivascular, and in collagenous areas. To investigate whether
there were differences in the deposition of C1q, we did manual
semiquantitation (Table 3). Only some of the short-term-IC
and IS NHPs inoculated with BbN40 showed increased C1q deposition by light
microscopy. However, dot-blot densitometry showed that the amount of C1q was
significantly higher in IS than in any of the two groups of TISP-NHPs (Table
6).
To investigate whether antibody and C1q deposition was
associated with deposition of the membrane attack complex (MAC/C5b-9), we did
immunohistochemistry with an anti-human MAC primary antibody. The results
showed the presence of MAC not only in the membranes from cardiac myocytes (Figure
4c) but also in spirochetes (Figure 4e). Detailed
examination of the MAC-stained spirochetes suggested that many appeared intact
while others appeared mildly damaged or degraded. To investigate whether MAC
deposition increased in the heart as a result of infection, we compared MAC
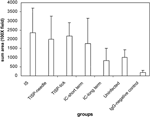
deposition by digital image analysis. The results (Figure 5)
showed significantly increased MAC in all groups of inoculated NHPs compared
with uninfected controls. The three highest values were for TISP-needle, TISP-tick
and IS-BbN40-inoculated NHPs. Mild but significantly increased MAC deposition
was also found in the garinii NHPs compared with uninfected controls.
To confirm whether MAC was being deposited in spirochetes as
suggested by immunohistochemistry, we did double immunofluorescence staining.
The results showed colocalization of MAC and Borrelia proteins on spirochetes
(Figure 4b, d, f). Examination of heart sections from IS-NHPs
revealed that there were both MAC-positive and MAC-negative spirochetes
throughout. These results showed that in steroid-treated NHPs heavily infected
with BbN40 MAC binds to but does not kill spirochetes.
B-Lymphocyte Chemoattractant (BLC)
The previous experiments demonstrated extensive
accumulation of plasma cells and antibody in the hearts of NHPs with Lyme
carditis. To investigate whether specific B-cell chemokines were being
produced in the hearts as a result of the infection that could be responsible
for plasma cell infiltration, we looked for the presence of the B-cell
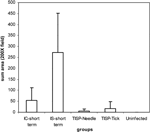
chemokine BLC/CXCL13. Digital image analysis of formalin-fixed immunostained
sections showed significant accumulation of BLC/CXCL13 mainly in IS and to a
lesser extent in IC-short-term and TISP-NHPs (Figure 6).
To make sure the signal from the anti-BLC/CXCL13 antibody was specific, we
repeated the immunostaining with and without blocking with BLC peptide. The
results (not shown) confirmed the anti-BLC antibody was specific.
Top of page
Discussion
This manuscript presents the first comprehensive investigation
of cardiac involvement in Lyme borreliosis in primates. The main findings of
the study were as follows: (1) carditis is very common in NHPs infected with B.
burgdorferi but is mild unless the animals are immunosuppressed. (2) The
spirochetal load in the heart is very high in NHPs necropsied while
immunosuppressed but decreased to minimal or undetectable in all NHPs
necropsied while immunocompetent. (3) The cellular inflammatory response to
the infection was characterized by multifocal collections of T cells, plasma
cells, and macrophages. (4) Infection resulted in increased deposition of IgG
and IgM in the heart. (5) Expression of the B-cell chemokine BLC was increased
accordingly to the spirochetal load. (6) Increased deposition of the
complement membrane attack complex (MAC) was found in the heart from TISP and
IS-NHPs, and a significant percentage of the spirochetes in the heart had MAC
on their membranes.
A previous report from our group described significant cardiac
inflammation and tissue injury in the heart of immunosuppressed NHPs infected
with the BbN40 strain.6 We had also seen
extensive cardiac injury in the heart of mice with severe combined
immunodeficiency infected with B. turicatae.18
In humans with Lyme disease, carditis is found in up to 25%, but only rarely
pathology specimens are available for examination.19
In NHPs we found that all but one inoculated with the BbN40 strain had
evidence of carditis at necropsy, including some that had been inoculated
years before. However, carditis was overall mild. The pattern of inflammation
was multifocal and patchy, although occasional large lesions were found (color
Figure 1a).
All groups of BbN40-inoculated NHPs had similar macrophage
infiltration with the exception of the long-term-IC group (Figure
2). The finding of increased numbers of T cells, plasma cells (Table
3, Figure 1c and d), and macrophages (Figure
2) in the heart is consistent with previous observations in small animal
models of Lyme carditis.20, 21,
22 Plasma cells were more abundant in IS-NHPs (Table
3), which was also the group with the highest expression of BLC (Figure
6) and the highest tissue deposition of IgM (Figure 4,
panel b). This suggests that one of the consequences of persistent B.
burgdorferi infection of the heart is upregulation of the B-cell chemokine
BLC leading to infiltration by plasma cells and production and deposition of
large amounts of IgM. The specificity of the IgM antibody deposited in heart
tissue has not been determined. It is also unknown why this IgM antibody is
unable to kill the spirochetes, as shown by the very high spirochetal load
present in the heart of IS-NHPs (Table 5).
BLC (also called BCA-1 or CXCL13) is a chemokine thought to be
especially selective for B-cells. BLC is considered a homing chemokine and has
been implicated in the trafficking of lymphocytes and dentritic cells in
lymphoid organs, and is critical for lymphoid neogenesis23
and for establishment of lymphoid follicle-like areas in chronically inflamed
tissues such as salivary glands in Sjogren's syndrome24
or joins in rheumatoid arthritis.25 In a
previous study, we found significantly increased levels of BLC mRNA in
skeletal muscle from TISP-NHPs inoculated with BbN40 compared with controls
that were uninfected or inoculated but not infected.26
As expected, a dramatic effect of the immunosuppression was an
inability to fight the infection. One reason why IS-NHPs failed to control the
infection was the lower levels of circulating specific antibody, as previously
reported.5, 7 Consistent
with this are the results of the dot-blot analysis (Table 6)
that showed higher total IgG in the heart of TISP compared with IS-NHPs.
Although digital image analysis showed similar levels of IgG, this is a less
sensitive technique than the dot blot. In contrast to IgG, IS-NHPs had much
higher IgM as shown both by dot blot and digital image analysis. As discussed
above, it is unclear why this IgM antibody failed to control the infection.
Another possibility for the higher spirochetal load in
steroid-treated animals is impaired complement activation that is required for
efficient spirochetal killing. In the absence of specific antibody B.
burgdorferi is resistant to the bactericidal activity of complement.27
Bactericidal antibody appears necessary for the effective formation of MAC.27
Most pathogenic microorganisms, and in particular those that circulate in the
blood stream like spirochetes, develop a wide range of strategies to elute
antibody and complement killing. We found that a large percentage of
spirochetes in the heart of IS-NHPs had MAC on their membranes (color Figure
4e), including many that appear morphologically intact. The reason why
spirochetes appear to survive MAC deposition in IS-NHPs is not known. One
possibility is disabling the correct assembly of MAC. MAC is an
ultrastructurally heterogenous complex that induces the formation of membrane
channels of different sizes.28 Patarakul et
al29 found similar level of MAC on the
membrane of a complement-resistant B. burgdorferi (WT297) and a
complement-sensitive mutant (MUT297). Although both had polymerization of C9
and MAC diffusely distributed and tightly bound on the outer membrane,
protease treatment rendered WT297 but not MUT297 susceptible to serum killing.
Proteins of 20, 30, and 66 kDa were found in the membrane of WT297 but
not in MUT297 that may be responsible for complement resistance.29
Two type of proteins that may be involved in complement resistance in B.
burgdorferi have been reported. One of them is OspE/Erp proteins that bind
factor H.30, 31 More
recently, a CD59-like molecule that inhibits the assembly of MAC was described
on the outer membrane of B. burgdorferi.32
We found deposition of MAC in the heart of IS-NHPs not only on
spirochetes but also on the membranes of cardiac myocytes. In Chagas
cardiomyopathy, another form of infectious carditis caused by parasite Trypanosoma
cruzi, MAC was found in the sarcolemma of 38% of cases compared with 0% of
controls.33 MAC deposition is also a feature
of damaged myocytes in myocardial infarction.34
We propose that in Lyme carditis complement activation in response to the
spirochetal infection leads to MAC deposition in cell membranes of cardiac
myositis with secondary fiber degeneration and fibrosis.
The true prevalence of Lyme carditis in humans is difficult to
determine because only few cases have been examined at autopsy. The incidence
of symptomatic Lyme carditis has been estimated to be 4–10% in adults.
However, the incidence of abnormal ECG findings in asymptomatic patients with
probable or definite Lyme borreliosis is higher, 29% in one study in children.35
The clinical course of Lyme carditis is usually benign with most patients
recovering completely. In rare instances, death has been reported.19,
36 The cardinal manifestation is conduction system
disease, which generally is self-limited. Heart block occurs usually at the
level of the atrioventricular node but often is unresponsive to atropine
sulfate. Temporary pacing may be necessary in more than 30% of patients, but
permanent heart block rarely develops. Myocardial and pericardial involvement
can occur but generally is mild and self-limited.37
Cardiomyopathy has been associated with B. burgdorferi infection in
Europe but not in the United States.4 No
treatment has been shown clearly to attenuate or prevent the development of
Lyme carditis, but mild carditis generally is treated with oral antibiotics
and severe carditis with intravenous antibiotics.37
Studies in mouse models of Lyme borreliosis showed that Lyme
carditis is very frequent but until now the true incidence in primates was not
known. Histopathological examination of mice with severe combined (scid)38
or other (NIH-3)39 immunodeficiency inoculated
with B. burgdorferi show high prevalence of severe carditis. Lyme
carditis is also prominent in immunocompetent mice20,
21 and is worse in IL-4-deficient mice.22
In C3H mice, spirochetes have been found in the heart as early as 6 days after
inoculation and all mice of the C3H and C57BL/6 haplotypes had infected
hearts.20 The spirochetes had a predilection
for connective tissue in the heart base. Carditis was first detectable on day
10, peaked on day 15, and resolved except for persistence of periaortic
lymphoplasmacytic infiltrates in all mice. The C3H mice developed more severe
disease than the C57BL/6 mice, and this was associated with earlier
appearance, greater numbers, and later clearance of spirochetes in C3H mice.20
A previous study of rhesus macaques inoculated with the JD-1 strain of B.
burgdorferi found focal myocarditis in three out of six hearts examined at
necropsy 6 months later.40
In summary, this study revealed Lyme carditis is very common
in infected NHPs when examined pathologically. Although severe infection of
the heart occurs in the setting of immunosuppression, the intact immune
response of NHPs reduced the infection to minimal or undetectable levels. The
failure of steroid-treated NHPs to clear the infection may be the result of
impaired killing due to decreased production of specific antibody or failure
of MAC assembly on the membranes of spirochetes.
Top of page
References
-
Steere AC. Lyme disease. N Engl J Med
2001;345:115–125 (see comments). | Article | PubMed | ISI | ChemPort |
-
Lyme disease—United States, 2000. Morb Mortal
Wkly Rep 2002;51:29–31.
-
Nagi KS, Joshi R, Thakur RK. Cardiac manifestations of Lyme disease: a
review. Can J Cardiol 1996;12:503–506. | PubMed | ISI | ChemPort |
-
Haddad FA, Nadelman RB. Lyme disease and the heart. Front
Biosci 2003;8:s769–s782. | PubMed | ISI |
-
Bai Y, Narayan K, Dail D, et al. Spinal cord involvement in the
nonhuman primate model of Lyme disease. Lab Invest
2004;84:160–172. | Article | PubMed | ISI | ChemPort |
-
Cadavid D, O'Neill T, Schaefer H, Pachner AR. Localization of Borrelia
burgdorferi in the nervous system and other organs in a nonhuman
primate model of lyme disease. Lab Invest
2000;80:1043–1054. | Article | PubMed | ISI | ChemPort |
-
Cadavid D, Bai Y, Dail D, et al. Infection and inflammation in
skeletal muscle from nonhuman primates infected with different genospecies
of the Lyme disease spirochete Borrelia burgdorferi. Infect
Immun 2003;71:7087–7098. | Article | PubMed | ISI | ChemPort |
-
Pachner AR, Delaney E, O'Neill T. Neuroborreliosis in the nonhuman
primate: Borrelia burgdorferi persists in the central nervous
system. Ann Neurol 1995;38:667–669. | PubMed | ISI | ChemPort |
-
Pachner AR, Delaney E, O'Neill T, et al. Inoculation of
nonhuman primates with the N40 strain of Borrelia burgdorferi leads
to a model of Lyme neuroborreliosis faithful to the human disease. Neurology
1995;45:165–172. | PubMed | ISI | ChemPort |
-
Pachner AR, Schaefer H, Amemiya K, et al. Pathogenesis of
neuroborreliosis—lessons from a monkey model. Wien
Klin Wochenschr 1998;110:870–873. | PubMed | ISI | ChemPort |
-
Moody KD, Barthold SW, Terwilliger GA. Lyme borreliosis in laboratory
animals: effect of host species and in vitro passage of Borrelia
burgdorferi. Am J Trop Med Hyg 1990;43:87–92. | PubMed | ISI | ChemPort |
-
Masuzawa T, Beppu Y, Kawabata H, et al. Experimental Borrelia
burgdorferi infection of outbred mice. J Clin
Microbiol 1992;30:3016–3018. | PubMed | ISI | ChemPort |
-
Busch U, Hizo-Teufel C, Boehmer R, et al. Three species of Borrelia
burgdorferi sensu lato (B. burgdorferi sensu stricto, B.
afzelii, and B. garinii) identified from cerebrospinal fluid
isolates by pulsed-field gel electrophoresis and PCR. J
Clin Microbiol 1996;34:1072–1078. | PubMed | ISI | ChemPort |
-
Pachner AR, Delaney E, Zhang WF, et al. Protection from Lyme
neuroborreliosis in nonhuman primates with a multiantigenic vaccine. Clin
Immunol 1999;91:310–313. | Article | PubMed | ISI | ChemPort |
-
Pachner AR, Braswell ST, Delaney E, et al. A rabbit model of
Lyme neuroborreliosis: characterization by PCR, serology, and sequencing
of the OspA gene from the brain. Neurology
1994;44:1938–1943. | PubMed | ISI | ChemPort |
-
Pachner AR, Cadavid D, Shu G, et al. Central and peripheral
nervous system infection, immunity, and inflammation in the NHP model of
Lyme borreliosis. Ann Neurol 2001;50:330–338. | Article | PubMed | ISI | ChemPort |
-
Pachner AR, Amemiya K, Bartlett M, et al. Lyme borreliosis in
rhesus macaques: effects of corticosteroids on spirochetal load and
isotype switching of anti-Borrelia burgdorferi antibody. Clin
Diagn Lab Immunol 2001;8:225–232. | Article | PubMed | ISI | ChemPort |
-
Cadavid D, Thomas DD, Crawley R, et al. Variability of a
bacterial surface protein and disease expression in a possible mouse model
of systemic Lyme borreliosis. J Exp Med 1994;179:631–642. | Article | PubMed | ISI | ChemPort |
-
Cary NR, Fox B, Wright DJ, et al. Fatal Lyme carditis and
endodermal heterotopia of the atrioventricular node. Postgrad
Med J 1990;66:134–136. | PubMed | ISI | ChemPort |
-
Armstrong AL, Barthold SW, Persing DH, et al. Carditis in Lyme
disease susceptible and resistant strains of laboratory mice infected with
Borrelia burgdorferi. Am J Trop Med Hyg
1992;47:249–258. | PubMed | ISI | ChemPort |
-
Ruderman EM, Kerr JS, Telford III SR, et al. Early murine Lyme
carditis has a macrophage predominance and is independent of major
histocompatibility complex class II-CD4+ T cell interactions. J
Infect Dis 1995;171:362–370. | PubMed | ISI | ChemPort |
-
Satoskar AR, Elizondo J, Monteforte GM, et al.
Interleukin-4-deficient BALB/c mice develop an enhanced Th1-like response
but control cardiac inflammation following Borrelia burgdorferi
infection. FEMS Microbiol Lett 2000;183:319–325. | Article | PubMed | ISI | ChemPort |
-
Ruddle NH. Lymphoid neo-organogenesis: lymphotoxin's role in
inflammation and development. Immunol Res
1999;19:119–125. | PubMed | ISI | ChemPort |
-
Amft N, Curnow SJ, Scheel-Toellner D, et al. Ectopic expression
of the B cell-attracting chemokine BCA-1 (CXCL13) on endothelial cells and
within lymphoid follicles contributes to the establishment of germinal
center-like structures in Sjogren's syndrome. Arthritis
Rheum 2001;44:2633–2641. | Article | PubMed | ISI | ChemPort |
-
Takemura S, Braun A, Crowson C, et al. Lymphoid neogenesis in
rheumatoid synovitis. J Immunol 2001;167:1072–1080. | PubMed | ISI | ChemPort |
-
Pachner AR, Dail D, Narayan K, et al. Increased expression of
B-lymphocyte chemoattractant, but not pro-inflammatory cytokines, in
muscle tissue in rhesus chronic Lyme borreliosis. Cytokine
2002;19:297–307. | Article | PubMed | ISI | ChemPort |
-
Kochi SK, Johnson RC, Dalmasso AP. Complement-mediated killing of the
Lyme disease spirochete Borrelia burgdorferi. Role of antibody in
formation of an effective membrane attack complex. J
Immunol 1991;146:3964–3970. | PubMed | ISI | ChemPort |
-
Tschopp J. Ultrastructure of the membrane attack complex of
complement. Heterogeneity of the complex caused by different degree of C9
polymerization. J Biol Chem 1984;259:7857–7863. | PubMed | ISI | ChemPort |
-
Patarakul K, Cole MF, Hughes CA. Complement resistance in Borrelia
burgdorferi strain 297: outer membrane proteins prevent MAC formation
at lysis susceptible sites. Microb Pathogen
1999;27:25–41. | Article | ISI | ChemPort |
-
Hellwage J, Meri T, Heikkila T, et al. The complement regulator
factor H binds to the surface protein OspE of Borrelia burgdorferi.
J Biol Chem 2001;276:8427–8435. | Article | PubMed | ISI | ChemPort |
-
Stevenson B, El-Hage N, Hines MA, et al. Differential binding
of host complement inhibitor factor H by Borrelia burgdorferi Erp
surface proteins: a possible mechanism underlying the expansive host range
of Lyme disease spirochetes. Infect Immun
2002;70:491–497. | Article | PubMed | ISI | ChemPort |
-
Pausa M, Pellis V, Cinco M, et al. Serum-resistant strains of Borrelia
burgdorferi evade complement-mediated killing by expressing a
CD59-like complement inhibitory molecule. J Immunol
2003;170:3214–3222. | PubMed | ISI | ChemPort |
-
Aiello VD, Reis MM, Benvenuti LA, et al. A possible role for
complement in the pathogenesis of chronic chagasic cardiomyopathy. J
Pathol 2002;197:224–229. | Article | PubMed | ISI | ChemPort |
-
Yasojima K, Schwab C, McGeer EG, et al. Human heart generates
complement proteins that are upregulated and activated after myocardial
infarction. Circ Res 1998;83:860–869. | PubMed | ISI | ChemPort |
-
Woolf PK, Lorsung EM, Edwards KS, et al. Electrocardiographic
findings in children with Lyme disease. Pediatr
Emerg Care 1991;7:334–336. | PubMed | ChemPort |
-
Marcus LC, Steere AC, Duray PH, et al. Fatal pancarditis in a
patient with coexistent Lyme disease and babesiosis. Demonstration of
spirochetes in the myocardium. Ann Intern Med
1985;103:374–376. | PubMed | ISI | ChemPort |
-
Pinto DS. Cardiac manifestations of Lyme disease. Med
Clin North Am 2002;86:285–296. | PubMed | ISI |
-
Schaible UE, Kramer MD, Justus CW, et al. Demonstration of
antigen-specific T cells and histopathological alterations in mice
experimentally inoculated with Borrelia burgdorferi. Infect
Immun 1989;57:41–47. | PubMed | ISI | ChemPort |
-
Defosse DL, Duray PH, Johnson RC. The NIH-3 immunodeficient mouse is a
model for Lyme borreliosis myositis and carditis. Am
J Pathol 1992;141:3–10. | PubMed | ISI | ChemPort |
-
Roberts ED, Bohm Jr RP, Cogswell FB, et al. Chronic lyme
disease in the rhesus monkey. Lab Invest
1995;72:146–160. | PubMed | ISI | ChemPort |
Top of page
Acknowledgements
This work was supported by Contract DMID-99-03 from the
NIH-NIAID. Dr Cadavid is a recipient of a Scientist Development Grant from the
American Heart Association-Heritage Affiliate. We thank Dr Bettina Wilske (Max
Von Pettenkofer Institut fur Hygiene und Medizinische Mikrobiologie, Munich,
Germany) and Dr Martin Schriefer (CDC, Fort Collins, Colorado) for providing
the B. garinii isolates. The assistance of the staff at the California
Primate Research Center is greatly appreciated.
FROM:
Cardiac involvement in non-human primates infected with the Lyme
disease spirochete Borrelia burgdorferi
Diego Cadavid, Yunhong Bai, Emir Hodzic, Kavitha Narayan,
Steven W Barthold and Andrew R Pachner
BACK
TO ARTICLE
|
![]() m
and cryomatrix sections at 8
m
and cryomatrix sections at 8 ![]() m.
Inflammation was assessed by examination of hematoxylin and eosin
(H&E) staining. To compare the severity of inflammation in heart,
H&E-stained sections were graded by a masked examiner for the
severity of inflammation per
m.
Inflammation was assessed by examination of hematoxylin and eosin
(H&E) staining. To compare the severity of inflammation in heart,
H&E-stained sections were graded by a masked examiner for the
severity of inflammation per ![]() 40 microscopic field as follows: absent inflammation=0; 1–3
foci=1+(minimal); 4–10 foci=2+(mild); 11–20 foci=3+(moderate); and
more than 20 foci: 4+(severe).
40 microscopic field as follows: absent inflammation=0; 1–3
foci=1+(minimal); 4–10 foci=2+(mild); 11–20 foci=3+(moderate); and
more than 20 foci: 4+(severe).![]() 40,
40, ![]() 100, or
100, or ![]() 200- magnification. The mean (s.d.) sum area (in square microns) and sum
optical density (in arbitrary units) were determined and compared
between groups. The intensity of immunostaining for some markers (CD3,
P63, and C1q) was compared manually by semiquantitation of the intensity
of the staining as absent (0), mild (1), moderate (2), or severe (3) by
a masked examiner.
200- magnification. The mean (s.d.) sum area (in square microns) and sum
optical density (in arbitrary units) were determined and compared
between groups. The intensity of immunostaining for some markers (CD3,
P63, and C1q) was compared manually by semiquantitation of the intensity
of the staining as absent (0), mild (1), moderate (2), or severe (3) by
a masked examiner.![]() l
reaction volumes. Taqman RT-PCR for the 16S rRNA of Borrelia spp.
was performed as described.5, 7
PCR-ELISA for the OspA or OspB B. burgdorferi genes was performed
as described.6 All assays were run in
triplicate.
l
reaction volumes. Taqman RT-PCR for the 16S rRNA of Borrelia spp.
was performed as described.5, 7
PCR-ELISA for the OspA or OspB B. burgdorferi genes was performed
as described.6 All assays were run in
triplicate.![]() g
in duplicate from each protein extract to polyvinylidene difluoride
membranes (Millipore). The primary antibodies were rabbit polyclonal
anti human IgG (Dako) or IgM at a 1:5000 dilution. The secondary
antibody was alkaline-phosphatase-conjugated goat anti-rabbit IgG
(Sigma). After incubation in fluorescence substrate ECF (Amersham's
RPN5785) for 5 min, the membranes were scanned with the Typhoon
8600 (Amershan Pharmacia Biotech Inc.). Results were analyzed by
densitometry using Image-Quant Software and expressed as mean (s.d.).
g
in duplicate from each protein extract to polyvinylidene difluoride
membranes (Millipore). The primary antibodies were rabbit polyclonal
anti human IgG (Dako) or IgM at a 1:5000 dilution. The secondary
antibody was alkaline-phosphatase-conjugated goat anti-rabbit IgG
(Sigma). After incubation in fluorescence substrate ECF (Amersham's
RPN5785) for 5 min, the membranes were scanned with the Typhoon
8600 (Amershan Pharmacia Biotech Inc.). Results were analyzed by
densitometry using Image-Quant Software and expressed as mean (s.d.). Full
table
Full
table
![]() 40 microscopic field was 2638 (2231), 12 327 (11 351), 13 762
(8176), and 21 246 (24 807) for uninfected controls, IS, TISP-tick,
and IC-short term NHPs, respectively (P-value <0.01 for all
groups compared with uninfected controls).
40 microscopic field was 2638 (2231), 12 327 (11 351), 13 762
(8176), and 21 246 (24 807) for uninfected controls, IS, TISP-tick,
and IC-short term NHPs, respectively (P-value <0.01 for all
groups compared with uninfected controls).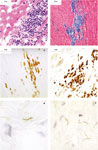
![]() 400); Trichrome staining in 372 IS-NHPs heart (panel b,
400); Trichrome staining in 372 IS-NHPs heart (panel b, ![]() 100); T cells in IS-NHP 372 heart (panel c, CD3 immunostaining
100); T cells in IS-NHP 372 heart (panel c, CD3 immunostaining ![]() 400); plasma cells in IS-NHP 1614 heart (panel d, P63
immunostaning
400); plasma cells in IS-NHP 1614 heart (panel d, P63
immunostaning ![]() 400); B. burgdorferi in the aorta (panel e) and atrium
(panel f) of IS NHP 571 inoculated with Bb297 (Borrelia
immunostaining
400); B. burgdorferi in the aorta (panel e) and atrium
(panel f) of IS NHP 571 inoculated with Bb297 (Borrelia
immunostaining ![]() 1000 in panel a and
1000 in panel a and ![]() 400 in panel f).
400 in panel f). EMPIRE STATE LYME DISEASE
ASSOCIATION
EMPIRE STATE LYME DISEASE
ASSOCIATION