|
|
|
*Home * About Us * Contact Us * Donations * Support Group Meetings * Links * JOIN US ! (free) *Archives *
|
|
|
Antimicrobial Agents and Chemotherapy, May 2008, p.
1728-1736, Vol. 52, No. 5 Persistence of Borrelia burgdorferi following Antibiotic
Treatment in Mice
Emir Hodzic, Sunlian Feng, Kevin Holden, Kimberly J. Freet, and
Stephen W. Barthold*
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The laboratory mouse is employed in extensively utilized models for Lyme borreliosis research. Mice develop many of the common manifestations of Lyme disease in humans, including arthritis, synovitis, carditis, myositis, and peripheral neuritis, but not the central nervous system disease or chronic unremitting arthritis that may occur in some human patients. In addition, the murine immune response to infection closely parallels that of humans (4). Studies of mice have revealed that spirochete numbers in tissues are highest during the early stage of infection and decline with the evolution of the host immune response (23). The evolution of host immunity results in global reduction of spirochete numbers in many tissues but fails to clear infection. In the persistent stage of infection, spirochetes are sequestered within highly collagenous areas of vessel walls, ligaments, and tendons. Spirochetes are also intercalated within collagen of the dermis, where ticks can acquire infection by feeding upon persistently infected mice (7). Evidence exists for persistence of spirochetes after antibiotic therapy in dogs, but the absence of clinical sequelae or culture positivity has raised questions about spirochete pathogenicity and viability (60, 61). Likewise, persisting B. burgdorferi infections in the ligamentous tissue (21), synovium (47), and skin (25, 45) of human patients following treatment with antibiotics have been documented.
In a study involving mice treated for 30 days with ceftriaxone or doxycycline, persistent infection was documented by detection of low levels of spirochetal DNA in tissues for up to 9 months and by feeding ticks upon the mice and then testing the ticks for spirochetes (xenodiagnosis). Infection could be detected by xenodiagnosis for up to 3 months after antibiotic treatment. Efforts to transmit infection to naïve mice from the infected ticks that fed on antibiotic-treated mice were not successful. Examination of the spirochetal DNA within positive-testing ticks suggested that the spirochetes had possibly lost genes or plasmids that were crucial for infectivity. It has been suggested that persisting spirochetes are attenuated and noninfectious (9). In another study using short-term ceftriaxone or ampicillin treatment in mice, spirochetal DNA could be detected in joint tissue but not in other tissues at 4 weeks after antibiotic treatment, but viable spirochetes could not be cultured (70). These results parallel those determined in studies of dogs treated with azithromycin, doxycycline, or ceftriaxone (61). The inability to culture spirochetes from DNA-positive tissues or to transmit spirochetes from infected ticks that fed on antibiotic-treated mice may be due to attenuation of spirochetes by antibiotics, as has been suggested, or may be due to the presence of a subpopulation of antibiotic-tolerant, nondividing "persister" bacteria (37, 55).
The current study further investigated the issue of B. burgdorferi persistence following antibiotic therapy by examining mice treated with ceftriaxone during the early stage of infection compared to mice treated during the late stage of infection. A recent study has shown that there are significant shifts into or preferential survival of spirochetes in collagen during chronic infection (7), which may facilitate immune evasion and impact effectiveness of antibiotics.
Borrelia burgdorferi. A low-passage-number clonal strain of B. burgdorferi N40 (cN40) sensu stricto was grown in modified Barbour-Stoenner-Kelly (BSKII) medium, as described previously (2). Mice were infected by subdermal inoculation of 104 mid-log-phase spirochetes in 0.1 ml of BSKII medium on the dorsal thoracic midline. The infection status of mice was assessed by culture of inoculation site, urinary bladder, and quadriceps muscle tissue, as previously described (5). Infection was also assessed by PCR amplification of B. burgdorferi target genes in samples of ear, skin, heart base, ventricular muscle, tibiotarsal joint, and quadriceps muscle tissue (see below). The sensitivity of BSKII medium for detection of viable spirochetes was verified by serial 10-fold dilutions of a B. burgdorferi N40 culture. Triplicate cultures were inoculated with 105, 104, 103, 102, 101, 100, and 10–1 dilutions of spirochetes and examined for growth at 5 and 12 days. Spirochete growth occurred at all dilutions through 100, indicating the high degree of sensitivity of spirochete growth in BSKII medium.
Antibiotic. Mice were treated with 16 mg/kg ceftriaxone in a 500-µl total volume of 0.9% normal saline solution administered intraperitoneally twice daily for 5 days and then once daily for 25 days. Sham-treated mice received a similar volume of saline solution. This dose has been previously shown to reach effective serum concentrations in mice following subcutaneous injection (9, 34). Serum ceftriaxone levels were evaluated in mice injected intraperitoneally with 16 mg/kg ceftriaxone-500 µl of saline solution and bled at 0, 1, 2, 4, 8, 12, and 24 h after treatment. Serum ceftriaxone levels were measured in triplicate 20-µl samples by use of an agar-based Staphylococcus aureus (ATCC 25923) inhibition assay, as described previously (34). Serum levels of ceftriaxone were 93 µg/ml, 20 µg/ml, and 2 µg/ml at 1, 2, and 4 h, respectively. No inhibition was detectable at 8 h or thereafter. These serum levels of ceftriaxone exceeded those measured in previous studies of mice given 16 mg/kg ceftriaxone subcutaneously (9, 34) and of dogs administered 25 mg/kg ceftriaxone intravenously (61).
The MIC and minimum bactericidal concentration (MBC) of ceftriaxone for B. burgdorferi N40 were determined to be 0.015 µg/ml (MIC) and 0.06 µg/ml (MBC), which are comparable to published MIC/MBC values of ceftriaxone for B. burgdorferi B31 (37, 55). Spirochetes (1 x 105 cells) were inoculated into 5-ml aliquots of liquid BSKII media prepared with serial 10-fold dilutions (100 µg/ml to 0.001 µg/ml) of ceftriaxone. The MIC was defined as the lowest concentration of ceftriaxone resulting in no visible growth of bacteria following incubation at 34°C for 3 days. Afterwards, 100 µl from each MIC culture was subcultured into 5 ml of BSKII media without antibiotics to determine the MBC, which was defined as the lowest corresponding concentration of ceftriaxone from the MIC cultures that demonstrated no visible growth following incubation at 34°C for 14 days. Tubes containing B. burgdorferi N40 without antibiotic and uninoculated media served as controls.
Ticks. Ticks used in this study were members of a single population of larvae derived from a colony of laboratory-reared pathogen-free Ixodes scapularis ticks and were provided by Durland Fish of Yale University. Several days prior to necropsy, each of the mice was infested with approximately 40 larval ticks, which were allowed to feed to repletion. Engorged larvae were collected and allowed to molt into nymphs and harden, and then randomly selected members of tick cohorts from each mouse were tested for the presence of B. burgdorferi by PCR (xenodiagnosis). Nymphal ticks from these cohorts were used for transmission studies.
PCR analysis. Spirochetal DNA was assessed by real-time quantitative PCR (Q-PCR), which was standardized and optimized for flagellin (flaB), outer surface protein A (ospA), and arthritis-related protein (arp), as described previously (23, 24). In addition, primers and an internal probe were developed for B. burgdorferi N40 vlsE. The B. burgdorferi N40 vlsE sequence has not been published, and primers based upon the B31 sequence failed to amplify vlsE from N40 DNA (data not shown). Therefore, an N40-specific sequence was obtained from an immunoreactive clone derived from screening an N40 genomic expression library with immune serum from infected mice, as described previously (14). The clone encoded a 318-bp fragment in which the first 100 bp shared 77% identity with B31 vlsE IR6 (vlsE 880-980) in addition to sequences that shared similarities of identity with 14 fragments within 18940-26270 on B31 linear plasmid 28-1 (lp28-1), where vlsE cassettes are located (11). From this clone, we designed a forward primer within the first 100-bp region (5'-TGATATGAAGAAGAAGGATAAGGTTGCT), a reverse primer that was downstream from this fragment (3'-TGTTGGTAAGAAGGAGGATGTACTAAAA), and an internal probe (GGATTGGCTAAAGATGGGAAGTTTTCGGTTACTAAT). The Q-PCR assay was optimized and all samples were assayed with positive and negative controls, as described previously (23, 24). DNA was extracted from tissues or ticks by use of DNeasy kits (Qiagen, Valencia, CA) according to the manufacturer's instructions for tissue or insects, respectively. Prior to DNA extraction, tissue samples were weighed, snap-frozen in liquid nitrogen, pulverized, and homogenized. Quantitative data were expressed as the number of DNA copies per milligram of tissue or the number of DNA copies per tick.
Serology. Antibody titers against B. burgdorferi were assayed by enzyme linked immunosorbent assay (ELISA), as described previously (15). Ninety-six-well microtiter plates (Nunc ImmunoMax Maxi-Sorp, Wiesbaden, Germany) were coated with 1 µg/ml B. burgdorferi cN40 lysate in carbonate buffer (pH 9.6) and incubated overnight at 4°C. Coated plates were washed with phosphate-buffered saline-Tween 20 and blocked for 1 h with 1% bovine serum albumin, and then duplicate serial threefold dilutions of serum samples (starting at 1:100 dilution) were added to each well. Plates were again incubated overnight, washed, and then incubated for 2 h with alkaline phosphatase-conjugated rat anti-mouse immunoglobulin (heavy and light chain) diluted 1:5,000. Alkaline phosphatase substrate (Sigma, St. Louis, MO) (1 mg/ml) was then added to each well for color development. Optical density was measured at a test wavelength of 405 nm with an ELISA reader (Molecular Devices Corporation, Sunnyvale, CA). Each assay included positive and negative controls. Cutoff points for each dilution were established by testing absorbance of normal mouse serum samples, determining means, and adding 3 standard deviations above the means.
Histology and immunohistochemistry. Formalin-fixed rear legs (demineralized in acid) and hearts were paraffin embedded and sectioned at 5 µm. Sections for histology were stained with hematoxylin and eosin. Legs were examined microscopically for evidence of arthritis and synovitis involving the knee and tibiotarsal joints as characterized by examination of infiltration of neutrophils, synovial proliferation, and exudation of fibrin into joint or tendon sheath lumina as well as neutrophil and macrophage infiltration of tissues at the base of the heart (carditis), as described in studies of C3H and C3H-scid mice (1, 4, 5, 8). Sections for immunohistochemical labeling of B. burgdorferi were processed using rabbit immune serum diluted 1:1,000 and biotinylated goat anti-rabbit immunoglobulin G (Vector Laboratories, Burlingame, CA), as described previously (7). Sections were randomly numbered and examined under blinded conditions without knowledge of treatment details.
Statistical analysis. Statistical comparisons between treatment groups and time points were performed, using Student's t test or chi-square analysis. Multiple comparison analyses were performed with one-way analysis of variance followed by a least-difference post hoc test (StatView, PowerPC version; SAS Institute Inc., Cary, NC). Calculated P values lower than 0.05 were considered significant.
Experimental design. The effectiveness of ceftriaxone or sham treatment was evaluated in mice following treatment that was commenced during the early (3 weeks) or late (4 months) stages of infection. These intervals were chosen to reflect two distinctly different phases of infection in the mouse model. The 3-week interval represented the early stage of infection during which the host immune response is evolving and disease (arthritis and carditis) is at its peak. The 4-month interval represented the persistent phase of infection, when host immunity is fully evolved and disease is resolved (5). Furthermore, spirochetes were sequestered in collagen during the late stage, making them potentially less vulnerable to antibiotic. Mice were subjected to necropsy at 1 and 3 months after completion of the 30-day treatment regimen at these two intervals. Infection status at necropsy was determined by culture and flaB DNA Q-PCR of tissues. In addition, infection status of mice was evaluated by xenodiagnosis, in which larval ticks were allowed to feed on mice several days prior to necropsy. Following feeding, molting, and hardening, nymphal ticks were tested for infection by flaB DNA Q-PCR. An attempt was made to obtain infectious spirochetes in ear tissues of mice by allograft transplantation of pieces of ear pinnae from antibiotic- or saline solution-treated mice into the subcutis of a single naïve C3H mouse. The recipient mice were subjected to necropsy, and tissues (urinary bladder, inoculation site, and quadriceps) were cultured and tested by flaB DNA Q-PCR (ear, inoculation site, heart base, ventricular muscle, tibiotarsus, and quadriceps tissue) at 3 weeks after allograft transplantation.
A confirmatory experiment was performed that examined antibiotic treatment during late infection. Mice were infected for 4 months, treated with antibiotic or saline solution, and then subjected to necropsy at 1 month after completion of treatment. In contrast to the initial study, additional tissue sites, including heart base, urinary bladder, ear, inoculation site, and tibiotarsus, were cultured. An attempt was made to increase the sensitivity of DNA detection using Q-PCR that targeted ospA DNA in lieu of flaB DNA, since ospA has been shown to be overrepresented relative to other targets (target imbalance) in tissue samples (42). PCR was performed using samples from additional tissue sites as well, including the inoculation site, subinoculation site (subcutis), heart base, ventricular muscle, tibiotarsus, quadriceps muscle, and ear. In addition, C3H-scid mice were utilized as recipients of allografts in lieu of C3H mice, and the recipient mice were assessed for infection at 3 weeks after transplant.
| View this table: [in a new window] |
TABLE 1. Culture, flaB DNA Q-PCR,
xenodiagnosis, allograft, and immunohistochemistry results for
individual mice treated with ceftriaxone or saline solution during
early infection (3 weeks) and subjected to necropsy at 1 or 3
months after completion of treatment
|
| View this table: [in a new window] |
TABLE 2. Culture, flaB DNA Q-PCR,
xenodiagnosis, allograft, and immunohistochemistry results for
individual mice treated with ceftriaxone or saline solution during
late infection (4 months) and subjected to necropsy at 1 or 3
months after completion of treatment
|
Immunohistochemical labeling of persisting spirochetes in tissues. Immunohistochemistry confirmed the PCR findings and further demonstrated that intact antigen-positive organisms with spirochetal morphology, rather than merely DNA, were present in tissues (Table 1 and Table 2). Heart and tibiotarsal joint tissue sections were examined under blinded conditions for the presence of antigen-expressing spirochetes. Small numbers (one to four per site) of spirochetes could be visualized in collagenous tissues (Fig. 1) of one or more mice in all treatment groups, with the exception of the results seen with mice treated with antibiotic during early infection and examined at 1 month after treatment. Among the antibiotic-treated mice, single spirochetes were typically localized to collagenous tissue of the great vessels at the base of the heart and tendons or ligaments of the joints. Up to four spirochetes were visualized in heart and joint sections of saline solution-treated mice, and they were also occasionally found in the interstitium of the ventricular myocardium of saline solution-treated but not antibiotic-treated mice.
 View larger version (102K): [in a new window] |
FIG. 1. Immunohistochemical labeling of
multiple B. burgdorferi spirochetes (arrows) in ligament
tissue of the tibiotarsal joint of a saline solution-treated
(control) mouse (A) and of a single spirochete (arrow) in ligament
tissue of the tibiotarsal joint of a ceftriaxone-treated mouse
(B). Both images are from mice treated with saline solution (A) or
ceftriaxone (B) at 4 months after inoculation with B.
burgdorferi and subjected to necropsy 1 month after completion
of treatment.
|
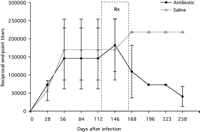 View larger version (17K): [in a new window] |
FIG. 2. B. burgdorferi
immunoglobulin G ELISA titers (mean reciprocal dilution ± SD) for
mice treated with saline solution or ceftriaxone for 1 month,
commencing at 4 months after infection. The dotted box (Rx)
indicates the interval of treatment relative to the stage of
infection.
|
| View this table: [in a new window] |
TABLE 3. Culture, ospA DNA Q-PCR,
and xenodiagnosis results for individual mice treated with
ceftriaxone or saline during late infection (4 months) and then
examined 1 month after completion of treatment
|
Tick-borne transmission of persisting spirochetes from antibiotic-treated mice to SCID mice. In a further effort to isolate spirochetes from xenodiagnosis DNA-positive ticks from tick pools obtained from the confirmatory experiment, we infested C3H-scid mice with eight nymphal ticks from cohorts of ticks derived from xenodiagnosis DNA-positive C3H mice. Based upon results of culture of urinary bladder, inoculation site, and quadriceps muscle tissue at 3 weeks after feeding, 0 of 10 C3H-scid mice became infected following feeding by ticks from antibiotic-treated mice, in contrast to four of four mice that became infected following infestation with ticks derived from saline solution-treated mice.
In an effort to extend these findings, each of nine C3H-scid mice was infested with 15 to 20 nymphal ticks from xenodiagnosis DNA-positive cohorts of ticks derived from mice that were treated with antibiotics (Table 4). In order to verify that the SCID mice were actually exposed to DNA-positive ticks, the infection status of each of the ticks that were used to feed upon the SCID mice was determined by flaB Q-PCR after ticks fed to repletion, molted, and hardened. The infection status of the SCID mice was assessed by culture of urinary bladder, ear, and inoculation site tissue and by flaB DNA Q-PCR of ear, subinoculation site, heart base, and tibiotarsus tissue. Mice were subjected to necropsy at 3 weeks after tick feeding. With the exception of one ear sample, all sites were culture negative. The exception was a culture tube that contained a single nonmotile spirochete. In contrast, flaB Q-PCR results indicated that one or more tissue samples from eight of nine SCID mice were positive. Copy numbers were generally low, ranging from as few as 2.8 flaB DNA copies/mg tissue to as many as 50,500/mg tissue (excluding the 50,500/mg value, the mean was 656.2 flaB DNA copies/mg ± 1,175.1 [SD]). Thus, it appeared that C3H-scid mice supported somewhat higher populations of spirochetes than C3H mice, and yet we were unable to culture spirochetes from tissues. Finally, joints (knees and tibiotarsi) and hearts of the SCID mice were examined for histopathology. None of the SCID mouse tissues showed microscopic evidence of inflammation despite the high prevalence of B. burgdorferi ospA DNA in hearts and joints and the propensity of infected C3H-scid mice to develop progressive severe inflammation (8).
| View this table: [in a new window] |
TABLE 4. flaB DNA QT-PCR,
PCR,
and culture results for individual SCID mice at 3 weeks following
feeding by ticks that fed upon mice treated with antibiotic during
late infection (4 months)
|
Because these conclusions are based upon DNA amplification, it could be argued that results do not absolutely prove the presence of live spirochetes. However, it has been shown that B. burgdorferi DNA does not persist in tissues unless live spirochetes are present (32, 42). We attempted to isolate spirochetes in order to characterize their plasmid content, but this was prevented by the inability of spirochetes to replicate in culture, despite the sensitivity of our culture method for detecting low numbers of spirochetes. This result was reinforced by the finding of a culture from an antibiotic-treated tick-infected C3H-scid mouse that contained a single nonmotile spirochete. In addition, the low levels of spirochetal DNA precluded detection of RNA transcription despite attempts to amplify cDNA (data not shown). We therefore undertook an exhaustive search for morphologically intact, antigen-expressing spirochetes in tissues by immunohistochemistry. There is increasing evidence that collagen is a critical niche for spirochete survival and possibly immune evasion (7, 30). We have found that vessels at the base of the heart and in ligaments and tendons of the tibiotarsal region (as well as in other joint tissues) are collagen-rich preferential regions for spirochetes in the persistent phase of infection (7). When these sites were examined by immunohistochemistry in the present study, low numbers of spirochetes were visualized in collagenous tissues of both antibiotic- and saline solution-treated mice. In C3H-scid mice infected with ticks from antibiotic-treated mice, the heart base and tibiotarsus tissues were also consistently positive by PCR. These findings support the idea of the viability of spirochetes following antibiotic treatment and further incriminate collagen as a preferential site of persistence that may contribute to antibiotic treatment failure. A study in which mice were treated with ceftriaxone for only 5 days resulted in similar findings, with detection of persisting spirochetal DNA in joint tissue (70). Persistence of Borrelia burgdorferi following antibiotic treatment of human patients in studies investigating collagenous tissue, including ligamentous tissue (21), synovium tissue (47), and skin tissue (25, 45), has also been documented.
The study by Bockenstedt et al. (9) utilized standard PCR to determine whether critical infectivity-associated plasmids or genes were present to explain the attenuation of spirochetes. The presence of lp25 of B. burgdorferi B31 has been shown to be essential for infectivity (49). The essential component has been defined as a single gene on lp25 that encodes a nicotinamidase (48). In addition, it appears that determinants encoded on lp28-1 of B. burgdorferi B31 affect relative infectivity (27, 28, 49). The lp28-1 plasmid of B. burgdorferi B31 encodes two functional genes, vlsE and arp (p37-47) in addition to a number of paralogous genes that are encoded on other plasmids or chromosomes, pseudogenes, genes with frame shift mutations, or sequences that encode products that are consist of fewer than 100 amino acids (16). Bockenstedt et al. (9) used standard PCR to evaluate the presence of BBE21.1, which is specific for lp25, and arp (p37-47), which is specific for lp28-1, and were unable to consistently amplify these targets in infected ticks that fed on antibiotic-treated mice. We tested DNA from three flaB DNA-positive ticks that fed on antibiotic-treated mice for the lp25 target and confirmed its presence. Since lp25 appears to be absolutely essential for infectivity and since our studies demonstrated the presence of infectivity, we focused on lp28-1 targets vlsE and arp. When B. burgdorferi N40-specific primers were used, both targets were present in all flaB DNA-positive samples tested by Q-PCR. It should be noted that the B. burgdorferi N40 genome is configured differently from the B. burgdorferi B31 genome and that vlsE and arp appear to be located on separate plasmids in B. burgdorferi N40 (S. Casjens, unpublished observations). Thus, using Q-PCR, which is more sensitive than standard PCR, we found no evidence of missing vlsE or arp. Loss of other plasmids has also been associated with diminished infectivity (33, 53, 68). Although we did not survey the entire genome, we found no evidence of spirochetes missing the critical infectivity-related genes that are homologous to B. burgdorferi B31 lp25 and lp28-1 in tissue samples from either mice or ticks.
There are a number of possible variables that may have influenced persistence of spirochetes in tissues following ceftriaxone treatment in this study. B. burgdorferi N40 has been shown to differ from other B. burgdorferi strains by being resistant to erythromycin compared to B. burgdorferi strains B31 and 297 (64), but B. burgdorferi N40 is as susceptible as B. burgdorferi B31 to ceftriaxone, as determined based upon MIC/MBC analysis in this study and by others (37, 55). Furthermore, serum levels of ceftriaxone in treated mice were well above the MIC/MBC. Nevertheless, the pharmacokinetics of ceftriaxone in mice differ from that of humans, as the trough levels in serum of treated humans are approximately 15 µg/ml at 24 h (43), whereas they were undetectable in mice at 8 h. Thus, the treatment regimen used for the mice, albeit long term, created a pulsed-dose effect. Studies with Enterococcus faecalis have shown that pulsed doses of penicillin resulted in development of bacterial tolerance to antibiotic without changes in resistance, whereas continuous treatment did not (22). Ceftriaxone binds to carboxypeptidases, endopeptidases, and transpeptidases in the cytoplasmic membrane and thereby inhibits cell wall synthesis and cell division (26). Spirochetes within collagen, especially in the persistent phase of infection, may be in a metabolically dormant (nondividing) state, with minimal cell wall synthesis, and this may affect levels of tolerance of ceftriaxone. Similar "persister cells" have been documented in a variety of bacterial infections (29, 54). Our findings parallel those of Straubinger et al. (59, 61, 62), who noted that cultures were uniformly negative in PCR-positive tissues from antibiotic-treated dogs in spite of the fact that culture assays were typically more sensitive than PCR for detection of B. burgdorferi in tissues of untreated dogs. These results fit with the concept of persister cells that evade killing by antibiotics. Further studies are needed to determine whether persisting B. burgdorferi spirochetes can revert to a dividing, pathogenic state.
The current study indicated that accessible indices of treatment, such as culture or PCR of skin and serologic response, cannot be relied upon as markers for treatment success. A declining antibody response, as has been noted following antibiotic treatment in mice (9) as well as in antibiotic-treated dogs (61), occurs despite low levels of persisting spirochetes. Our results show that spirochetes are viable and transmissible and express antigen (based upon immunohistochemistry results) following antibiotic treatment, particularly when commenced during the late stage of infection. However, the few residual spirochetes appeared to be altered in their ability to replicate, and this may explain the lack of inflammation that we noted in SCID mouse tissues. Although overt disease may not be present, the continued expression of lipoproteins by B. burgdorferi may contribute to persistence of constitutional symptoms. Spirochetal lipoproteins have been shown to potently elicit a wide variety of proinflammatory responses (12, 13, 18, 19, 31, 36, 39, 40, 50, 57, 63, 65, 66, 69). This may explain the slow recovery period (termed "post-Lyme disease syndrome") that has been noted following antibiotic treatment of patients (67). Further studies are needed to determine the eventual fate of the persisting organisms following antibiotic treatment in the context of controlled animal studies.
The authors are indebted to Gary Wormser for editorial comments and to Durland Fish for provision of ticks used in this study.
![]() Published ahead of print on 3 March 2008.
Published ahead of print on 3 March 2008. ![]()
This article has been cited by other articles:
Copyright © 2010 by the American Society for Microbiology. For an alternate route to Journals.ASM.org, visit: http://intl-journals.asm.org | More Info»
| Abstract
|
||||||||||
| Full Text (PDF) | ||||||||||
| Alert me when this article is cited | ||||||||||
| Alert me if a correction is posted | ||||||||||
| Citation Map | ||||||||||
| E-mail this article to a friend | ||||||||||
| Similar articles in this journal | ||||||||||
| Similar articles in ASM journals | ||||||||||
| Similar articles in PubMed | ||||||||||
| Alert me to new issues of the journal | ||||||||||
| Download to citation manager | ||||||||||
| Reprints and Permissions | ||||||||||
| Copyright Information | ||||||||||
| Books from ASM Press | ||||||||||
| MicrobeWorld | ||||||||||
| Citing Articles via HighWire | ||||||||||
| Citing Articles via Google Scholar | ||||||||||
| Articles by Hodzic, E. | ||||||||||
| Articles by Barthold, S. W. | ||||||||||
| Search for Related Content | ||||||||||
| PubMed Citation | ||||||||||
| Articles by Hodzic, E. | ||||||||||
| Articles by Barthold, S. W. | ||||||||||
Pubmed/NCBI
databases
|
||||||||||